Shrimp Farm Technical Guidlines
Control Water Quality in Shrimp Farming
Good water quality by proper management will help prevent shrimp diseases and create an environment for better growth and higher survival rate.
|
Factor |
Measurement device |
Test time |
|
Secchi disk |
At 3 PM everyday |
|
|
Dissolved oxygen |
D.O. measuring probes / meters |
At 4 AM and 4 PM everyday |
|
pH test kit or pH meters |
At 4 AM and 4 PM everyday |
|
|
Refractometer |
Everyday |
|
|
test kit |
Every week |
|
|
Ammonia nitrate nitrite |
test kit |
Every week |
|
Sulfate |
test kit |
Once every two weeks |
|
Vibrio bacteria |
TCBS agar - test kit |
Every week |
|
Luminous Vibrio |
TCBS agar - test kit |
Every week |
|
Algae |
Microscope |
Every week |

pH of water
In aquaculture, changes in pH can affect physical elements, chemistry, and biology of water environment and health of the species.
- Appropriate pH for water in shrimp ponds is pH = 7.2 to 8.8. The best condition is from 7.8 to 8.5.
- pH fluctuation during a day should not exceed 0.5. If pH changes significantly, it can make shrimp and fish shocked, weakened and stop eating.
- If high or low pH extends for a long time, it will make shrimp grow slowly, stunting and susceptible to diseases
pH of water depends on these factors:
1 . Natural ground/platform of the ponds:
• Acid sulfate soil (acidic alum soil, acidic soil) makes pH of water low and fluctuated.
• If it rains a lot; aluminum is washed away from dikes into ponds; water soaking in dikes or water in trench leaking to ponds will increase the amount of aluminum in ponds and reduce pH.
• If we plow the alum ground, aluminum will blow up into the upper floors.
2 . Algae and microorganisms in lakes:
• Algae and microorganisms using CO2 can affect the pH of water.
• A large amount of algae will make pH strongly fluctuated during the day. Too much algae also makes pH high (8.8 to 9.1) in the afternoon.
• When algae dies, pH in ponds will be reduce.
• In shrimp areas whose salinity is low, or in rainy season, algae often increase.
• Balance between algae and microorganisms need to be maintained to stabilize pH.
pH in ponds often increases during the day and decreases at night. In Viet Linh’s experience, it is necessary to measure pH at least 2 times/day to monitor, identify causes of fluctuations and timely control situations.


Some simple solutions that can stabilize pH of water in aquaculture ponds:
1 . Handling bottom environment of the ponds:
Using quicklime or slaked lime to renovate ponds’ bottoms.
Check pH of the bottom soil. The lower the pH, the more we have used lime to increase pH.
• pH
> 6: use 300 - 600 kg of lime/ha
• pH < 5: use 1500 - 2000 kg of lime/ha
2 . Water Treatment:
2.1 . When pH is low:
• When pH drops too low, use lime, or slaked lime with a dose of 0.5 - 10 kg/1000 m2. Spread lime around the pond, about 10kg/1000m2 before it rains.
• For pH in the morning is from 7.5 to 7.8, and the difference in the afternoon exceeds 0.3: If the water is pure, use 30 - 50kg dolomite lime/1600 m2 (180-300 kg/ha) in the afternoon continously in 2-3 days.
• For pH in the morning is from 7.5 to 7.8, and the differences in the afternoon is 0.5: If the water color is normal, use CaCO3 clime of 180-300 kg/ha every afternoon until the pH stop fluctuate too much during the day.
2.2 In case of high pH
• If pH > 8.3 in the morning: use sugar with a dose of 1-3 kg/1000 m2, or use appropriate probiotics to stimulate the development of microbial decomposition. These microorganisms decompose organic detritus in the pond. CO2 will be produced and pH will be reduced.
• pH can be reduced by replacing less water.
• In case of a sudden increase in sunny afternoons, pH > 9.0, use formalin with a dose of 3-4 ml/m3.
If pH fluctuation is large during the day (> 0.5), hardness (the amount of CaCO3) in ponds is low, algae grows and develop quickly causing algal blooms, organic detritus increases in ponds, use dolimite lime with a dose of 100-200 kg/ha to increase water hardness and buffering agent. Water should also be changed to stabilize the growth of algae.

Alkalinity of water
Total alkalinity is the total amount of sodium bicarbonate (HCO3-) and carbonate (CO32-) in water, converted into mg/L (ppm) of calcium carbonate (CaCO3).
Water alkalinity for black tiger prawn farming:
• Newly
released prawn: 80 - 100ppm
• 45 days or older prawn: 100 - 130ppm
• 90 days or older prawn: 130-160 ppm
Water alkalinity for whiteleg shrimp farming:
•
Newly released shrimp: 100 - 120ppm
• 45 days or older shrimp: 120 - 150ppm
• 90 days or older shrimp: 150-200 ppm
If alkalinity is low, use lime or dolomite 30-50kg/1600m2 one time every 2-3 days until the pH reaches the required levels.
Alkalinity is a pH buffer that provides CO2 for photosynthesis of
algae and plants in water.
When water alkalinity is high, pH is more stable and less changeable.
Below pH 8.3, alkalinity is present mostly as bicarbonate (HCO3- )


Dissolved oxygen of water in shrimp pond
Dissolved oxygen (DO) is an important factor in aquaculture production. Low dissolved oxygen in the water can cause deaths in shrimp and fish.
|
Dissolved oxygen (ppm) |
Effects on shrimp |
|
0.3 |
Shrimp die |
|
1.0 |
Anoxia in shrimp, shrimp may die |
|
2.0 |
Shrimp cannot grow up |
|
3.0 |
Shrimp grows slowly |
|
4.0 |
Shrimp grows normally |
|
5.0 - 7.0 |
Shrimp grows healthily and rapidly |
Thus, the best levels of dissolved oxygen for shrimp are equal or higher than 5 ppm.
Oxygen concentration decreases depending on the depth of water. Dissolved oxygen decreases when temperature and salinity decrease.
During the day, with sunlight, algae and plankton photosynthesize and create oxygen dissolved in water.
At night, on days without sunlight or with overcast weather and rain, water will not have enough dissolved oxygen for shrimp. At this time, dissolved oxygen should be increased by using paddlewheel aerator, aeration blower, or changing a part of water.
Excessive use of chemicals to remove algae or insecticides that make aquatic plants die can also cause a shortage of dissolved oxygen.
Some symptoms of shrimp when water with dissolved oxygen is lacked: Shrimp concentrates near water surface, edges of ponds, near position where water comes in; lethargic shrimp with strong respiratory rate, coma and possible death in shrimp.
In water which is saturated air, dissolved air will invade the circulatory system of shrimp and form bubbles impeding shrimp blood flow. This creates "gas bubble disease" that causes deaths in shrimp.
The water is saturated air when:
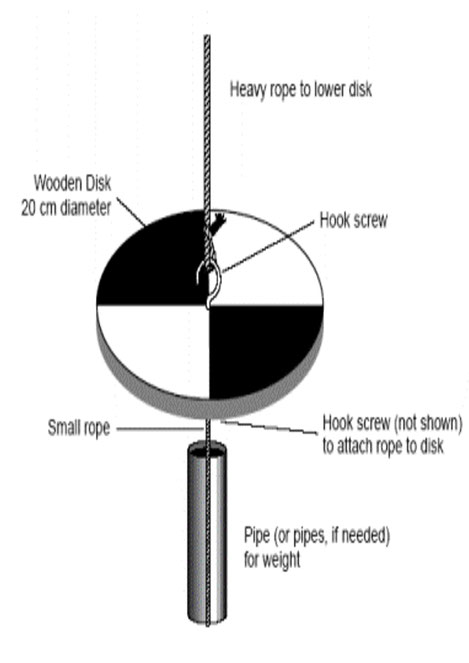
• Photosynthesis of phytoplankton is too much (transparency measured by Secchi disk is read at 10cm or less).
• The water temperature increased.
• Disturbance strong between water layers (at positions fan, water pump)
Overcome the lack of oxygen in the pond:
- Pond needs aeration. If drop waterhyacinth, duckweed or water spinach to make shelter when it's sunny, them gather together in a corner of the pond, do not drop more than 1/3 of the pond surface.
- Do not feed and fertilize with excess doses. This will reduce the concentration of dissolved oxygen. Died algae consumes oxygen and generates CO2, NH3, H2S...
- Change the water with other good quality water.
- When a mass of shrimp, fish swim on water surface and weak active (swimming lethargically, swimming near edges of ponds, no reaction to sound): need aeration, turn on water fan, supply new water.
- Use hydrogen peroxide H2O2: 2H2O2 → 2H2O + O2
When dissolved oxygen is less than 4 ppm, shrimps swim on the water surface: need more aeration and water changes; check and the adjustment feed intake; avoid fresh food, add vitamins, minerals and supplements to food; manage quality of water carefully.
If the dissolved oxygen is too low in morning and too high in afternoon, this makes algae thrive. We must control the feed, use 10 - 20kg CaCO3 / 1600 m2, turn on aeration in night, manage quality of water.
When the dissolved oxygen is too low, use more air blowers.



Algae in aquaculture water
In aquaculture water, there are 5 groups of microorganisms and algae:
• Algae
- phytoplankton (algae)
• Bacteria
• Fungal spores (fungi)
• Viruses
• Zooplankton
Common groups of algae in aquaculture ponds:
• Blue
green algae
• Dinoflagellate algae
• Diatom algae
• Green algae
The impacts of algae to aquatic life in ponds and lakes:
• Algae affect the balance of oxygen and CO2 in water:
- During the day, under the influence of sunlight, algae use CO2 as a material for photosynthesis that produces oxygen (O2).
- When there is no sunshine, rain and at night, algae use oxygen (O2) for respiration.
- Thus, the amount of dissolved oxygen (DO) in water fluctuates significantly: high at noon and low at down.
- Algae indirectly affect the pH value of water.
• Blue green algae: This group of algae is harmful to shrimp. Rakhoroni algae cause scum on the water surface, like Microcytis sp., it makes shrimp smell fishy and foul; it discharges gel through cell membrane, and cause blockage in shrimp’s gills.
• Dinoflagellate algae: There are many types of algae in this group conveying toxins that can make shrimp die.
• Diatom algae: diatoms are feed of post-larval (E.g.: Chaetoceros sp., Skeletonema sp.). The life cycle of algae in this group is relatively short, so they easily cause water color change.
• Green algae: this group contains no toxicity, usually has small size and doesn’t make shrimp smell. It has long lifetime, which can stabilize water color. Chlorella sp. algae are in this group.Chlorella sp. has the ability to prevent the growth of Vibrio bacteria.
The amount of algae and types of algae in the water can greatly affect color and turbidity of aquaculture water.

TAN = total ammonia nitrogen
In water, ammonia (NH3) exists in equilibrium with dissolved ammonium ions (NH4+).
TAN (total ammonia nitrogen) is the total amount of nitrogen in the forms of NH3 and NH4+ in water.
In aquaculture, TAN concentration must be less than 0.5 mg/L.
There are two processes using TAN in water by micro-organisms:
1. The assimilation process:
The heterotrophic bacteria assimilate TAN into microbial protein. This process should have both nitrogen and carbon sources.
According to some references of Viet Linh, the ratio C:N> 15 is considered to be a good rate for heterotrophic bacteria to consume TAN, and keep TAN concentration in water at low levels. In aquaculture model biofloc, biofloc particles include bacteria sticking with other organisms and organic particles. Biofloc particles that range from 0.1 to several mm are the source of food supplying protein for farmed fish and shrimp.
2. Nitrification converts TAN into nitrate:
In this transformation process, in the first step, TAN is transformed into nitrite (NO2-, toxic). After that, nitrite (NO2-) is converted into nitrate (NO3-). These two steps all require the participation of bacteria and oxygen.
To reduce ammonia (NH3) and nitrite (NO2-), Viet Linh suggests the fastest method is to change water. When water cannot be changed, supplement of organic carbon to farming ponds is necessary as it helps heterotrophic bacteria "digest" ammonia. Sugar and molasses are sources of organic carbon for water in ponds. Also, sufficient oxygen supporting activities of anaerobic bacteria, stable pH and densities algal-bacteria must also be ensured.

